New research by UNC-Chapel Hill and the University of California, San Diego, develops a low-cost test strip that provides a simple and accurate detection of COVID-19 infection within minutes. Its portability and ease of manufacture could make it globally available, especially in rural or low-income areas that typically lack easy access to expensive PCR testing equipment.
In a quickly evolving public health crisis, current methods for COVID-19 tracing and diagnosis fall short of the speed or accuracy needed for global disease containment, but now, an interdisciplinary team led by scientists from the University of North Carolina at Chapel Hill and the University of California, San Diego have designed a rapid and sensitive lateral flow assay that has the potential to become the gold standard for the detection of SARS-CoV-2 variants.

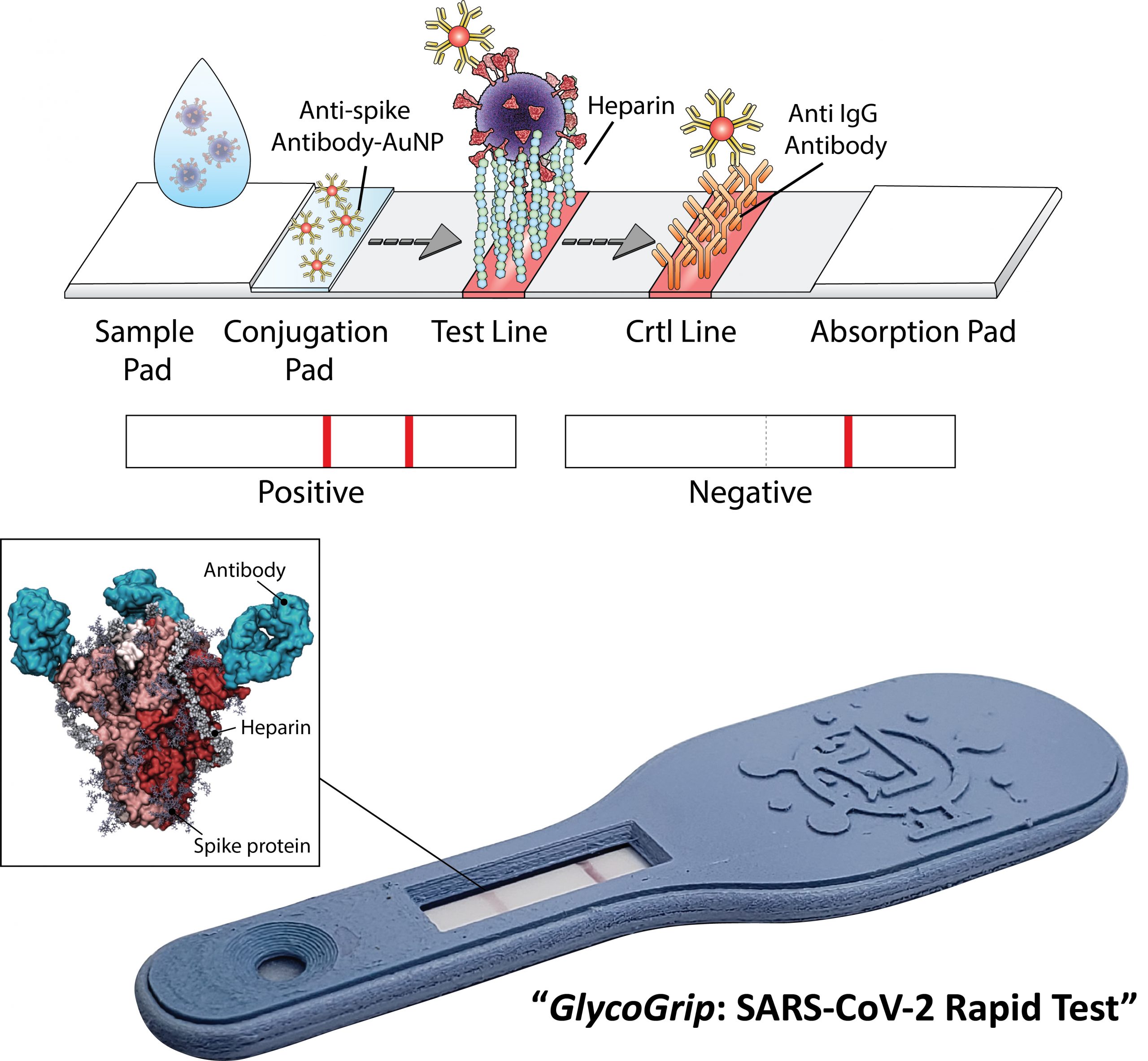
This new test, named GlycoGrip, adapts natural biology to reliably capture the SARS-CoV-2 virus and allow for a simple and accurate detection of COVID-19 infection within minutes. Its low cost, portability, and ease of manufacture could make it globally available, especially in rural or low-income areas that typically lack easy access to expensive PCR testing equipment.
The findings were published today (DATE) in the journal ACS Central Science.
“We tapped into nature to reimagine viral diagnostics,” said Ronit Freeman, co-corresponding author of the paper. Freeman is an associate professor of applied physical sciences and biomedical engineering in the College of Arts & Sciences at the University of North Carolina at Chapel Hill.
GlycoGrip is inspired by the natural biology of epithelial cells – those that are targeted and infiltrated by the SARS-CoV-2 virus. These cells are coated with a dense matrix of sugars called the glycocalyx, and it’s this sugar-net that the virus exploits to cause infection.
“We have turned the tables on the virus by using the same sugar coat it binds to infect cells – to capture it onto our sensor,” said Freeman. The concept is intuitive: a droplet of biofluid containing the virus, such as saliva, is placed on one end of the strip and flows along the surface. When the fluid reaches a sugar-coated patch, the virus can’t help but indulge its sweet tooth, becoming trapped on that specific area. This capture is then signaled by antibodies treated with gold nanoparticles producing a visual color that indicates infection.
To better understand how these sugar polymers bind the virus, Freeman connected with Rommie Amaro, a professor of chemistry and biochemistry at the University of California, San Diego and co-corresponding author. Amaro and her team developed computationally intensive simulations that helped explain the mechanics behind how and why the cell-anchored sugars bind the viral spikes. “By using atomic-level views of the spike protein, we were able to identify key binding sites for the glycocalyx sugar polymers and unlock how these sugars adapt to different spike conformations,” said Amaro. “This is exciting, we essentially revealed another secret of how spike binds cells to facilitate infection.”
One of the greatest challenges of the ongoing COVID-19 pandemic has been responding to the virus’ emerging variants. New tests must be developed for each new mutation. But GlycoGrip offers a solution. It has proven effective in testing for all known variants. “We are optimistic that GlycoGrip will capture future variants just as easily,” said Freeman. A patent has been filed for this new technology, and looking beyond the current pandemic, the team envisions a future in which GlycoGrip can offer cheap and reliable testing for a wide range of viruses.
The paper is titled “GlycoGrip: Cell Surface-inspired Universal Sensor for Betacoronaviruses.” The full author list includes: Sanghoon Kim, Fiona Kearns, Mia Rosenfeld, Lorenzo Casalino, Micah Papanikolas, Carlos Simmerling, Rommie E. Amaro and Ronit Freeman.
The research was funded by Research Corporation for Science Advancement (COVID Initiative grant #27350) award, the North Carolina Policy Collaboratory at the University of North Carolina at Chapel Hill with funding from the North Carolina Coronavirus Relief Fund established and appropriated by the North Carolina General Assembly, NSF RAPID (DMS-2028758, MCB-2032054), UNC Institute for Convergent Science Director’s Fund, NIH GM132826, and UC San Diego Moores Cancer Center 2020 SARS-COV-2 seed grant.
