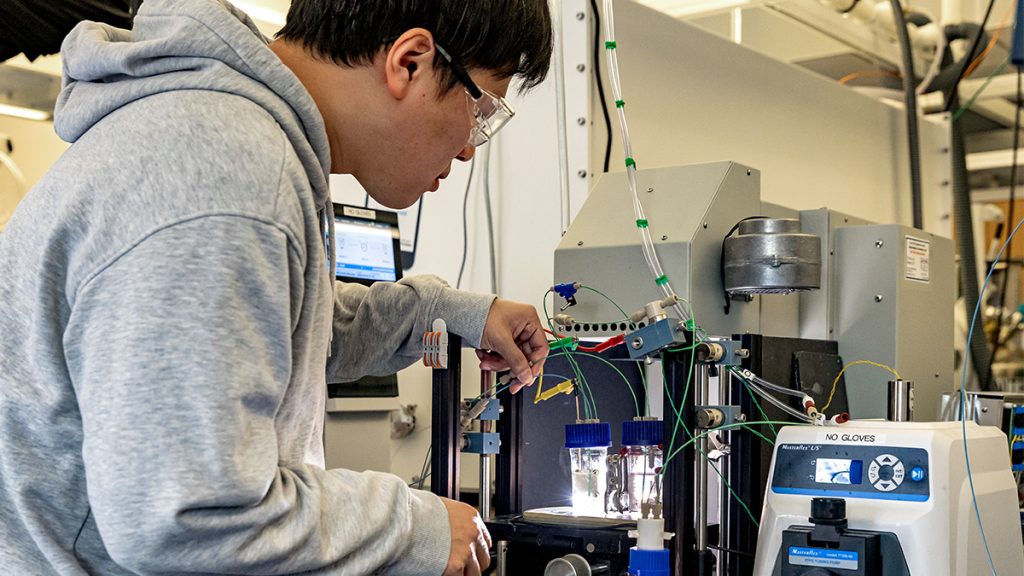
At the center’s Chapel Hill headquarters, more than 100 researchers work to turn sunlight into methanol.
The sun powers our solar system, lights our Earth and helps sustain life as we know it, yet much of the sun’s energy remains untapped by humans. As the world grapples with emerging climate and energy crises, scientists are increasingly looking toward the sun for solutions.
Carolina researchers have taken on a prominent role in the development of that science, especially since the 2020 launch of the Center for Hybrid Approaches in Solar Energy to Liquid Fuels in Chapel Hill.
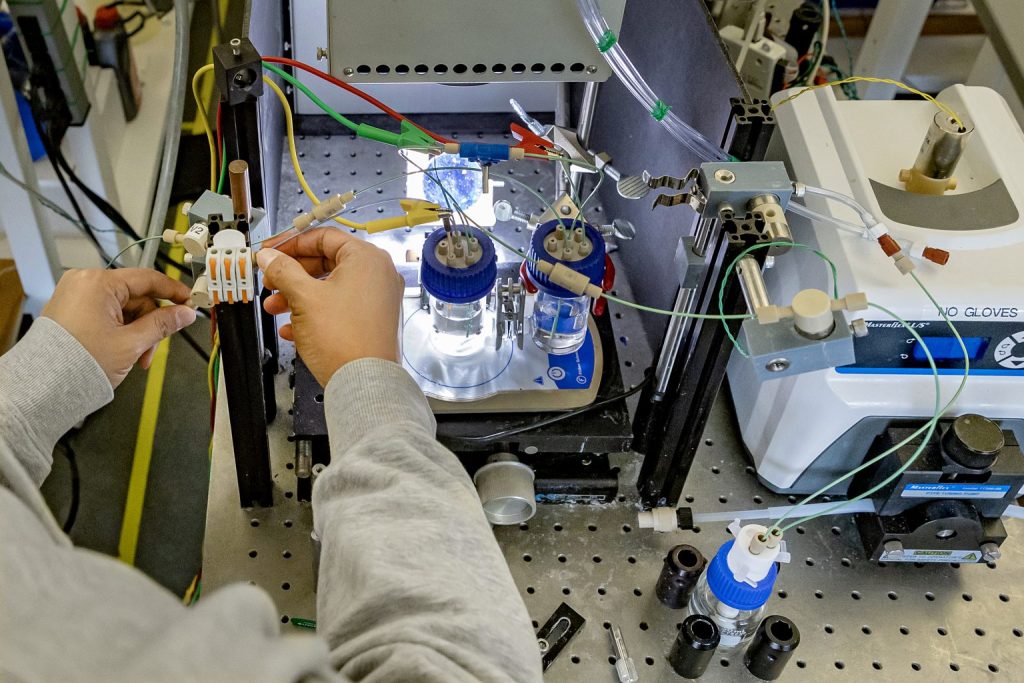
The CHASE Solar Hub, funded by a five-year, $40-million grant from the U.S. Department of Energy Office of Basic Energy Sciences, positions UNC-Chapel Hill as a national leader in solar energy research. Now in Year 4, CHASE has already made significant headway, including the development of three mechanisms that convert sunlight, water and carbon dioxide into liquid methanol.

“The big-picture goal of CHASE is to make meaningful progress in the science behind using sunlight to drive the conversion of energy-poor molecules — like carbon dioxide and water — into energy-rich fuel,” said Jillian Dempsey, CHASE deputy director and Bowman and Gordon Gray Distinguished Term Professor of chemistry in the College of Arts and Sciences.
“There’s obviously a major need to move away from fossil fuel energy sources toward sustainable energy sources to meet our growing energy needs and mitigate climate change.”
Despite the proliferation of solar panels and other photovoltaic devices, the conversion of sunlight into liquid fuel is a relatively undeveloped science. Because our energy economy runs on liquid fuel — and because liquid fuel has a much higher energy density than batteries — its production from sunlight could have massive global ramifications.
Carolina is working in partnership with six institutions — Yale, Princeton, Emory and NC State universities, the University of Pennsylvania and Brookhaven National Laboratory — to create a highly collaborative environment to solve a complex and multifaceted issue.
“We are not a single researcher working on this problem,” Dempsey said. “We are a hub of over 100 people from distinct backgrounds, distinct expertise and complementary expertise working on this problem.”
What makes liquid fuel production from sunlight so challenging?
Picture the gasoline in your car. When gasoline combusts, it produces carbon dioxide, water vapor and energy. What CHASE is trying to do, in essence, is to reverse that reaction and use carbon dioxide, water and energy to produce fuel.
This “uphill” chemical reaction does not occur spontaneously and requires energy. By pairing light-absorbing semiconductors with molecular catalysts, CHASE aims to harness that energy from the sun to make liquid fuels.
CHASE is focused on developing the science that could lay the foundations for future technology.
“One can imagine in the future having a closed-loop system, where you take a carbon-containing liquid fuel and burn it very much in the way we do now, but do it in a controlled way, in what’s called a fuel cell,” said Gerald Meyer, CHASE director and chemistry professor. “You could burn the liquid fuel, get the carbon dioxide and then recycle it without releasing it to the atmosphere.”
Such a system would create a more sustainable and renewable energy source while also mitigating the climate impact of carbon dioxide in the atmosphere. But there are still questions to answer and problems to solve before that kind of technology is available. With CHASE’s funding set to expire in 2025, both Meyer and Dempsey said the hope is for a grant renewal.
“We’re in Year 4, and we’re hopeful that we’ll be able to renew CHASE for another five years,” Dempsey said. “We want to push the science forward even further and bring it to that technology-readiness level.”
This research was supported as part of CHASE, an Energy Innovation Hub funded by the U.S. Department of Energy, Office of Science.
By Michael Lananna, University Communications
